 AMD is the leading cause of legal blindness in industrialized countries. It has two forms, atrophic and exudative, and a multifactorial pathogenesis. To cope with the ever-increasing incidence of AMD, retinal specialists resort to three strategies: primary prevention, patient management in clinical practice and prospective medical research aimed at finding new therapies.
AMD is the leading cause of legal blindness in industrialized countries. It has two forms, atrophic and exudative, and a multifactorial pathogenesis. To cope with the ever-increasing incidence of AMD, retinal specialists resort to three strategies: primary prevention, patient management in clinical practice and prospective medical research aimed at finding new therapies.
Age-related Macular Degeneration (AMD) is a chronic degenerative disease of the retina. It selectively affects the central portion of the retina called the macula and causes the degeneration of retinal visual cells. AMD is the leading cause of legal blindness in industrialized countries. The pathology affects people over 50, and leads to the progressive loss of central vision, which is essential for seeing the details required to perform such everyday tasks as reading, recognizing faces and driving.
Pathophysiological mechanisms
The exact pathophysiological mechanisms of AMD are still poorly elucidated, but the implication of intoxication processes leading to the death of retinal pigment epithelial cells has been established in recent years. During aging, these cells may present functional impairment related to the accumulation of proteolipid complexes, known as lipofuscin granules, in lysosomes. These granules are formed gradually by the accumulation of undegraded protein and lipids from the external photoreceptor fragments phagocytosed by the pigment epithelium. Lipofuscin also contains cytotoxic derivatives derived from the visual cycle such as A2E. Under the effect of blue light, A2E oxidizes and induces protein, lipid and DNA oxidation, causing significant oxidative stress in the cells of the retinal pigment epithelium during aging and resulting in the death of the latter.
Pathogenesis
Age-related degeneration of the macula has a multifactorial pathogenesis. The primary factor is, of course, age, since the disease appears after age 50 and its prevalence increases rapidly after age 75. There is also a genetic predisposition to the disease: the risk of developing AMD is four times greater if a parent or sibling has it. Several genetic polymorphisms associated with the disease have been identified. Among them, variants of the gene coding for complement factor H or the gene encoding HTRA1 (a protease) are implicated. Since 2005, a total of 19 loci have been identified as being related to AMD. They involve a variety of biological functions, including the regulation of the innate immune system, maintenance of cellular structure, growth and permeability of blood vessels, lipid metabolism and atherosclerosis. The simultaneous presence of three variants (factor H, HTRA1 and CC2-FB) in the same individual can increase the risk of developing AMD by a factor of up to 250.
Smoking is strongly associated with AMD: it increases the risk of developing the disease by a factor of three.
Numerous research studies have shown that a diet low in vitamins, trace elements and antioxidants can predispose to the disease.
Retinal phototoxicity related to blue light is also implicated in the pathogenesis of AMD. The wavelengths responsible for this toxicity in the presence of lipofuscin were recently elucidated in vitro revealing a spectrum of blue-violet light ranging from 415nm to 455nm with a highly toxic peak at 435nm. This toxicity increases in proportion to the amount of lipofuscin in the retina, but a slight toxicity remains, even in the absence of lipofuscin. These wavelengths are, of course, present in the solar spectrum, but can also be found in the radiation of certain light-emitting diodes.
Obesity also doubles the risk of AMD. Hypertension, cardiovascular diseases and cholesterol have been implicated as well, but their role remains uncertain.
The forms of AMD
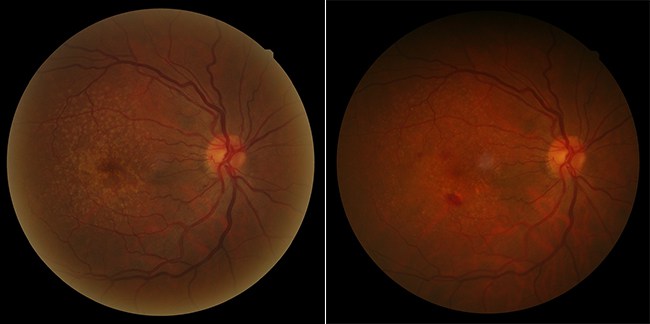
AMD has two different forms, atrophic and exudative. The atrophic or “dry” form is related to atrophy of the macula, characterized by the progressive degeneration of the retinal pigment epithelium and the neurosensory retina. There is no known cure for this form of the disease, which progresses slowly. The exudative or “wet” form is characterized by the abnormal development of blood vessels below the macula. This ocular neoangiogenesis is also known as choroidal or subretinal neovascularization, thus providing another name for this form: neovascular AMD. These malformed new blood vessels are fragile and porous, and are therefore subject to vascular hyperpermeability. They also destroy the normal architecture of the retina and its functioning.
The neovascular form has various subtypes depending on the type and location of neovasculature relative to the pigment epithelium. The photoreceptors suffer and ultimately scar tissue develops, permanently destroying the macula. This is the most aggressive form of the disease and may represent two-thirds of the forms of AMD. Choroidal neovascularization is due to the phenomenon of angiogenesis, in which vascular endothelial growth factor (VEGF) plays a significant role. VEGF has therefore been the target of new therapeutic strategies developed in recent years, leading to the development of anti- VEGF treatments. These treatments are now the gold standard for treating the disease and are administered via intravitreal (i.e. intraocular) injections, which are repeated every other month on average.
The early stages of AMD are characterized by the presence of small yellowish white spots on the fundus in the macula region called drusen and/or alterations of the pigment epithelium. This feature defines age-related maculopathy or pre-AMD.
Exudative AMD: treatment protocol
The severity and speed of development of the exudative form, along with the efficacy and cost of the treatments developed to date, make it a real public health concern and a diagnostic and therapeutic emergency.
Ophthalmologists/retinologists who treat AMD patients must be able to see them on very short notice (within a week at the most) if they present with scotoma (dark spots in their central vision) or macular syndrome: a decrease in visual acuity or difficulty reading; metamorphopsia (distorted perception of images and straight lines). In the presence of these symptoms, eye exams must be conducted promptly, including visual acuity assessment via an EDTRS chart, a fundus examination via a binocular slit lamp, fluorescein and/or indocyanine green (ICG) retinal angiography and optical coherence tomography (OCT).
If the diagnosis of subfoveal exudative AMD is confirmed by these examinations, it is recommended that anti-VEFG treatment be initiated as early as possible, irrespective of the initial level of visual acuity. Anti- VEFG treatments must be administered by intravitreous injection. Extra- and juxtafoveal choroidal neovascularization with subfoveal exudative manifestations should be considered a subfoveal location of AMD.
In the current state of science, the most commonly adopted treatment protocol is as follows: one anti-VEFG injection per month during three consecutive months (the interval between the two injections must be at least four weeks), followed by a monitoring phase. During the monitoring phase, patients must be examined every four weeks as follows: a visual acuity assessment using an ETDRS chart; a fundus examination and/or retinogram; an optical coherence tomogram (OCT). Fluorescein angiography may be performed if necessary.
Another injection must be given after the first three injections, if persistent or recurring signs of continued activity of the neovascular lesion are clinically detected via a fundus examination and/or optical coherence tomography. On average, patients receive six or seven injections per year.
AMD is a bilateral disease. After the first eye is affected, there is an increased risk of bilateralization (about 10% per year). In the presence of functional symptoms (i.e. visual impairment, metamorphopsia, scotoma, etc.) concerning the fellow eye during follow-up, the patient should be seen on an emergency basis. It is recommended that surveillance examinations for AMD be performed on both eyes to screen for an asymptomatic incipient lesion in the fellow eye.
Atrophic AMD: patient management
Unfortunately, the same therapeutic advances are not available for patients with atrophic AMD as for those with the exudative form.
Although it progresses more slowly, the long-term prognosis remains poor and possible complications involving neovascularization warrant regular monitoring (i.e. self-monitoring using the Amsler grid, leading to a rapid consultation if there is any change in functional symptoms).
When the decrease in visual acuity becomes debilitating, management of patients with advanced AMD involves rehabilitation and the use of low-vision-support magnifying optical systems to mobilize unaffected areas of retina to improve vision.
Patients presenting with age-related maculopathy must be educated about self-monitoring methods, via the Amsler grid in particular. Patients at very high risk with large confluent drusen and RPE alterations must be examined frequently to detect the possible appearance of neovascularization amenable to treatment. It is essential that patients receive a clear diagnosis. Ensure that patients know the name of the disease that is the cause of their declining visual acuity and whether they are suffering from an early or advanced stage of the atrophic or exudative form of the disease.
Explain to patients that this is a chronic condition that can be treated but cannot be cured, and that it does not lead to total blindness (since peripheral vision is preserved). Regular follow-ups are essential.
Inform patients about their visual prognosis, the risk of involvement of the second eye and the risk of progression from the atrophic form to the exudative form.
Primary prevention
The constantly increasing incidence of AMD fully justifies strong primary prevention efforts to combat this condition. Primary prevention is based on combating the risk factors for the disease. For example, simple lifestyle and dietary preventive measures can be recommended to all, such as: combating tobacco use; combating obesity, lipid disorders and hypertension; practicing a physical activity on a regular basis; and adopting a diet rich in the macular pigments lutein and zeaxanthin (found in fruits and vegetables) and omega 3s (oily fish like salmon, tuna, etc.).
Based on the results of conclusive research on the toxicity of blue-violet light radiation, a partnership with a manufacturer of eyeglass lenses has led to the development of lenses (Crizal ® Prevencia ®) capable of reflecting a fraction of this toxic radiation and preventing it from penetrating the eye. It is therefore logical to recommend that as many people as possible wear this type of photo-selective protection, particularly those with genetic risk factors.
Protection from solar radiation via photo-protective lenses is still called for out-of-doors from an early age in view of the transparency of the crystalline lens in children.
The prevention of complications for patients with precursor lesions is also currently necessary. This is accomplished primarily through the prescription of food supplements, on the basis of evidence provided by the publication of a number of large epidemiological studies. Supplementation with antioxidants (zinc and vitamins C and E) decreases the risk of progression and worsening of AMD by 25% in at-risk patients. Supplementation with 10mg of lutein and 2mg of zeaxanthin in addition to antioxidants would reduce the progression of advanced AMD by 18%. The value of omega 3 is not as clear-cut, but studies have shown that taking large doses of DHA could reduce the risk of developing neovascular AMD in high-risk patients.
The prescription of food supplements whose composition is consistent with data from these studies is therefore recommended for at-risk patients and patients known to have AMD.
New therapeutic avenues
A number of new therapeutic avenues for AMD are currently being explored.
Some new molecules should soon be available in association with anti-VEGF treatments for exudative AMD, including anti-PDGF (Platelet derived growth factor) agents, also administered via intraocular injection. Numerous other molecules are also being tested, including complement factor inhibitors and anti-TNF (tumor necrosis factor) molecules.
Gene therapy is also being studied in exudative AMD with the goal of producing an anti-VEGF agent directly in the retina by introducing a gene directly into retinal cells by a viral vector. This would free patients from risks related to repeated injections.
Cell therapy is a new avenue being explored for atrophic AMD. The idea is to implant stem cells or autologous RPE cells in the retina to renew the
supply of functional cells and stop the degenerative process. Lastly, for visually impaired patients at a very advanced stage, an artificial retina is also under development. An implant is placed in the retina that will receive images via an eyeglass-mounted camera.
Article from the magazine "Point de vue"


