 Light is suspected of being a risk factor for major vision-threatening diseases. Yet an equal light exposure can unequally affect people. Multiple intricate factors are responsible for a distinct personal risk profile. The scientific quest in understanding both eye phototoxicity and individual risk profiles can set a turning point towards personalized prevention in the future.
Light is suspected of being a risk factor for major vision-threatening diseases. Yet an equal light exposure can unequally affect people. Multiple intricate factors are responsible for a distinct personal risk profile. The scientific quest in understanding both eye phototoxicity and individual risk profiles can set a turning point towards personalized prevention in the future.
Each day, our retina absorbs millions of billions of photons with an expected increased magnitude due to our new light exposure behaviors. Day after day, these streams of photons can induce irre-versible eye damage and contribute to the onset or development of debilitating eye diseases. The phenomenon is aggravated by the accelerated ageing of the world population, since an ageing eye is more photosensitive along with altered defense.
A better understanding of the pathogenesis of vision-threatening diseases, a sharp analysis of light/eye interactions, and an individual risk profiling for these eye conditions are now urgent to provide appropriate and personalized eye photo-protection solutions, starting with eyewear, for efficient and long-term prevention.
1. EYE PHOTOTOXICITY
While light is necessary and beneficial to visual and non-visual functions, any optical radiation might potentially be hazardous to the eye if it is received and absorbed by eye tissues at doses capable of causing photomechanical, photothermal or photochemical reactions. On the one hand, brief and extreme bright light exposure may induce mechanical or thermal permanent and rapid eye injuries. On the other hand, moderate light exposure for an extended period of time may result in progressive biochemical changes and ultimately induce irreversible cell death. For this chronic lifelong eye light damage, the spectral specificity of light is critical. In particular, UV radiations and high-energy visible light are pointed out as high risk spectral bands respectively for the anterior eye and the retina.
UV radiations and the anterior eye

Chronic eye exposure to solar UV radiations has been progressively associated with the pathogenesis of numerous cornea and crystalline lens diseases. If additional photobiology studies would be of interest to better dissect the intricate link between UV and the eye, sufficient in vitro, in vivo and epidemiology data confirm the contributory role of UV in numerous diseases of the anterior eye, such as cataracts, pterygium, conjunctivitis, pinguecula, climatic droplet keratopathy, ocular surface squamous neoplasia, etc. (for more details, see Points de Vue no. 67).
In 1956, Kerkenezov observed an early clinical indication of the role of UV in pterygium. Later, Minas Coroneo evidenced that peripheral light focusing by the anterior eye to the sites of usual locations of pterygium and cataract is involved in the pathogenesis of these eye conditions. The Chesapeake Bay study reported a significant correlation between the spatial zone affected by the climatic droplet keratopathy and the average annual UV exposure. Corinne Dot et al. evidenced that mountain professionals are at higher risk for cataracts. The POLA, Beaver Dam Eye and Chesapeake Bay epidemiology studies revealed a higher prevalence of cortical cataracts in populations living in bright sunny plains.
Public awareness has rapidly been high on the UV eye hazard since skin UV protection has now long been encouraged and normalized (SPF factors).
Blue light and the retina
Since UV radiations are totally absorbed by the cornea and the crystalline lens after the age of 20, the most energetic light reaching the retina is blue light.
Photobiology studies on blue-light eye damage started half a century ago, with the landmark paper of Noell evidencing blue retinal phototoxicity in rodents exposed to white fluorescent lamps. In 1972, Marshall, Mellerio and Palmer observed blue light damage in the pigeon cones. Since then, with the advent of lasers, the number of photobiology studies on blue light has soared. Ophthalmologists themselves have been encouraging such phototoxicity and exposure threshold studies for their patients’ exposure when conducting laser surgery (for retinal procedures, also for refractive surgery) or for themselves considering the light intensity of ophthalmic instruments (slit-lamp and others). More recently, in the 1990s, the IOL industry has funded phototoxicity research to support the benefits and safety of the blue-light filtering IOLs implanted during cataract procedures.
In vivo experiments revealed that photochemical damages to the retina exhibit lower dose thresholds in the blue range compared to green and red as evidenced in monkeys, rats and rabbits.
Blue light hazards were further studied on the outer retina (photoreceptors and retinal pigment epithelium (RPE) (Fig. 1), on immortalized RPE cells loaded with either oxidized photoreceptor outer segment, purified lipofuscin or synthesized A2E. A greater toxicity of blue light was demonstrated by exposing human RPE loaded with lipofuscin during 48 hours upon violet-blue-green light (390 nm – 550 nm, 2.8 mW/cm²) and yellow-red light (550 nm – 800 nm, 2.8 mW/cm²). This cell death was mediated by apoptotic processes involving caspase-3 and p-53 activation.
Many of these studies suffer limitations such as not being precise enough on the light dose sent, or illuminating with very high irradiances that trigger acute light-toxicity mechanisms rather than lifelong cumulative exposure damage. Moderate irradiances and longer exposure should be sought when studying the pathogenic mechanisms of Age-Related Macular Degeneration (AMD) or diabetic reti-nopathy. Under the supervision of Professor Sahel and Dr. Picaud, Paris Vision Institute and Essilor researchers joined skills to go a step further from a photometry standpoint. By developing innovative cell illumination protocols and systems, we together have studied various phototoxic action spectra involved in the pathogenesis of severe vision-threatening diseases (AMD, retinitis pigmentosa, glaucoma, etc.). For instance, we have evidenced the precise phototoxic action spectrum of RPE within the blue-green range in sunlight physiological retinal exposure on an established in vitro model of AMD. The 415 nm - 455 nm narrow spectral range was highlighted as the greatest phototoxic risk to RPE cells (Fig. 2).
In vitro and in vivo studies have progressively revealed a strong scientific rationale for cumulative blue toxicity on the outer retina. The understanding of cell mechanisms involved has provided crucial inputs on the pathogenesis of outer retina diseases, in particular AMD. First, cumulative exposure to blue light favors the accumulation of all-trans-retinal in the photoreceptor outer segments (POS). All-trans-retinal interacts with blue-violet light with a decreasing profile between 400 nm and 450 nm. Its blue photo-activation induces oxidative stress within the POS. This stress is normally compensated by retinal antioxidants and enzymes, but age progressively reduces anti-oxidative defenses, thus failing to compensate for the oxidative stress. The POS progressively oxidize, and their renewal into the RPE is more challenging as their membrane components are difficult for the RPE to break down. Thus, intracellular digestion is incomplete and generates an accumulation of residual lipofuscin in the RPE. Lipofuscin is sensitive to blue-violet light. Blue photoactivation may generate reactive oxygen species. When the number of these species exceeds cellular defence capacity, RPE cells die by apoptosis. Deprived of these support cells, the photoreceptors deteriorate in turn, contributing to the loss of vision diagnosed in patients suffering from AMD. Age and light-related accumulation of lipofuscin in the RPE are major pathogenesis features of AMD.
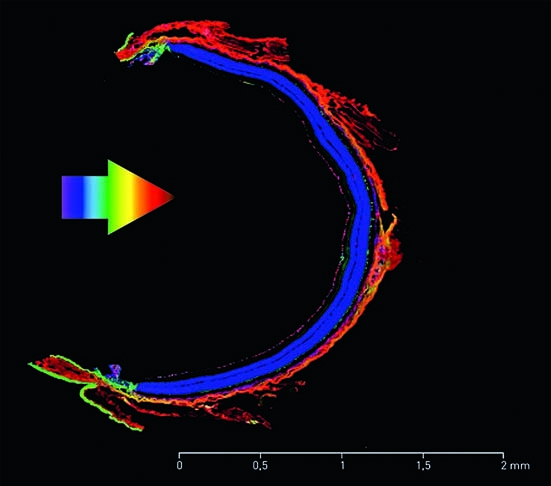
 Fig. 1: Retinal tissues Paris Vision Institute images by confocal microscopy. RGC=Retinal Ganglion Cells; IPL=Inner Plexiform Layer; INL=Inner Nuclear Layer; OPL=Outer Plexiform Layer; ONL=Outer Nuclear Layer; RPE=Retinal Pigment Epithelium
Fig. 1: Retinal tissues Paris Vision Institute images by confocal microscopy. RGC=Retinal Ganglion Cells; IPL=Inner Plexiform Layer; INL=Inner Nuclear Layer; OPL=Outer Plexiform Layer; ONL=Outer Nuclear Layer; RPE=Retinal Pigment Epithelium
Numerous epidemiology studies confirm the correlation between blue light exposure and AMD. The EUREYE study found significant association between blue light exposure and neovascular AMD in individuals having the lowest antioxidant level. In the Chesapeake Bay study performed on 838 watermen, AMD patients – compared with age-matched controls – were significantly higher exposed to blue over the preceding 20 years but equally exposed to UV, suggesting that blue light exposure is related to AMD. The Beaver Dam Eye Study reported a correlation between sunlight and 5-year incidence of early AMD changes. Leisure time spent outdoors while persons were teenagers (13 – 19 years) and in their 30s (30 – 39 years) was significantly associated with the risk of early age-related macular changes. A recent meta-analysis led by Sui et al. interestingly concluded that light is a risk factor for AMD.
Beyond the outer retina, photobiologists have recently suspected that high energy visible light could also affect inner layers of retina, such as retinal ganglion cells (RGC). Specific blue light may be absorbed by chromophores located in mitochondria. As an abundance of mitochondria are localized in RGC and these cells are involved in the degenerative processes of glaucoma, we suspect that blue light is a risk factor for glaucoma as well. In ageing retina where functional mitochondria are no longer in an optimum homeostatic state, blue light might dramatically precipitate the onset of glaucoma and other optic neuropathies. It could even contribute to accelerating glaucoma once diagnosed.
Light, a risk factor for major vision-threatening diseases
Light is suspected of being a risk factor in many debilitating eye diseases. For cataract and AMD, it is now well established: UV radiations accelerate the cataract onset while in AMD, blue-violet light exposure is a precipitating factor. For other diseases such as diabetic retinopathy or glaucoma, photobiologists suspect cumulative lifetime exposure to blue light contributes to the oxidative stress of specific retinal cells. In all cases, the contribution of light among other pathogenic factors grows with age and when the defence and repair mechanisms against photochemical damage are less effective, which is the case when the eye disease is already diagnosed (e.g. antioxidant enzymes such as SOD-2 or catalase are less effective).
Normative data
European and ISO standards for sunglasses (EN 1836 and ISO 12312-1) and for tinted ophthalmic lenses (ISO 8980-3) have, for many years, used a relative spectral effectiveness weighting function S(λ) to characterize UV hazards. This was originally published in ICNIRP guidelines 1989 and is derived from an action spectrum for skin erythema.
A sister function in the blue range was proposed later, B(λ), derived from the seminal work by Ham et al. for the acute hazard on aphakic monkey eyes. B(λ) was defined by multiplying the spectral values of Ham et al.’s research with the spectral transmittance of the human lens. Nevertheless, there is no standard on cumulative blue light toxicity. New identification of phototoxic action spectra [24] should be advantageously used to create and/ or revise normative data on phototoxicity.
From in vitro and in vivo… to clinical data
In vitro phototoxicity studies bring valuable and robust information on the light action spectrum as well as on the light-associated specific cell and disease biomarkers. The Essilor and Paris Vision Institute study with 10 nm step illuminations is a good illustration. Animal models (in vivo) are interesting living and integrative models of a disease. They make it possible to study the role of a specific gene (transgenic knock-out animals), the involvement of a specific toxicity pathway (e.g. inflammation, oxidative stress) or a disease target. They are essential in correlating disease with imaging, biology testing, immunohistochemistry or behaviour. But they have limitations: pathogenic mechanisms can differ from humans (e.g. using rodents in AMD models while rodents do not have a macula); light illumination is more intense and of a shorter duration than in real life [6] . While in vitro and in vivo experiments raise the understanding of a disease-specific light action spectrum and pathogenic mechanisms, the only clinical evidence of light-associated eye diseases is brought by longitudinal epidemiological studies. It is therefore necessary, when studying real-life eye chronic phototoxicity, to find light-specific markers of disease early-signs and disease progression.
2. INDIVIDUAL RISK PROFILING
Now that cataract is being better treated in eastern and southern countries, AMD, glaucoma and diabetic retinopathy are becoming the three major vision-threatening diseases. This threat is largely worsened by the world ageing and by lifestyle risk factors such as poor antioxidant diet. From a public health perspective, considering that it is now urgent to optimize the disease management for these three eye conditions, all efforts should be paid to raise awareness in the general population, especially in the sub-populations considered more at risk, to develop and select the most relevant tests for early diagnosis and disease profiling, ultimately to monitor the disease progress and to apply the most appropriate therapeutic strategy. This sounds obvious but we believe we have now reached a more mature stage of understanding of the disease mechanisms associated with the availability of a set of new diagnostic tools including eye imaging, biological testing of biomarkers and psychophysical methods. In order to manage effectively a multifactorial disease, there is an utmost need of disease profiling and monitoring and if applicable of multi-facetted therapy – aiming at multiple mechanistic targets – as well as prevention.
Individual risk profiling is defined by multiple intricate factors, source-, patient-and environment-dependent (Fig. 3).
Individual light exposure profile
Our own light exposure profile is defined by the correlation of the number of light sources, their local-ization, their spatial distribution, their radiance including directivity, but, critically, also their spectral distribution, the exposure duration and repetitions.
There is no doubt that solar radiations are the most harmful ones, since sun radiance is more than 100 times higher than the radiance of standard artificial lighting and since daylight is rich in UV and blue light. The physical environment (ground reflectances, altitude, latitude, etc.) significantly modifies the amount of light received by the eye. The eye UV dose increases by 10% every 1,000 metres. While sand reflects 10% of UVB, water reflects 20% and snow more than 80%. Therefore, populations exposed to bright sunlight in high ground reflectance environments (mountain professionals, etc.) or living in very sunny plains are at higher risk of UV and blue-light related eye damage, including cataracts and AMD.
In addition to daylight, in our ageing and connected digital world illuminated by new solid-state lighting, our light exposure profile is rapidly and dramatically evolving. Starting at increasingly younger ages of our existence, our eyes are subjected to longer and simultaneous exposures, at shorter distances, with higher radiance and higher energy than with former incandescent sources.
Since they produce light with much lower energy consumption, these new solid state lighting sources have become the dominant domestic lighting technology. In Europe, by 2016, no traditional incandescent light sources will be available. The European lighting industry estimates that over 90% of the total luminaires world market will be based on solid state lighting products by 2020. Beyond domestic lighting, the LED compactness plus the wide spectral range they can cover (monochromatic LEDs) have generated many new lighting applications, for mobile phone and tablet back lighting or even for toys and clothes.
New LED-based light sources may emit more blue than former incandescent lamps. Current white LEDs are combining a blue pumped LED with a phosphor emitting at higher wavelengths. For mass production of white LEDs, blue diodes based on InGaN or GaN crystals are combined with a yellow phosphor (YAG:Ce or similar); they produce “cold-white” with a color temperature CCT equal or higher than 5500 K . They may emit up to 35% of blue light within the visible range, much more than incandescent lamps (< 5%). To produce “warm-white” with a CCT <3200 K, with less than 10% of blue, an extra layer of phosphor emitting red light is needed, which significantly reduces the luminous efficacy of the LED.
At retinal level, received irradiance is directly proportional to the radiance of the light source. By having a small light emission area, LEDs have a higher radiance, which makes them brighter, even for the same irradiance level.
Worldwide initiatives have been launched to conduct a health risk assessment on systems using LEDs. A task group was for instance mandated by the French Agency for Food, Environmental and Occupational Health & Safety (ANSES) in 2008. They concluded that a photochemical blue light risk could exist, consecutive to prolonged white LED exposure. Risky light exposure profiles may be identified and related to high-risk populations (for more details, see Points de Vue no. 68):
- the daily adjustment and testing of high power cold white LEDs, by lighting installers, operators in lighting manufacturing facilities, show technicians and collectors, dentists, surgeons, etc.;
- the use of toys with LEDs, since children have a crystalline lens more transparent to blue light;
- automotive LED daytime running lights, when activated near children or photosensitive persons (aphake, pseudo-aphake eyes, people suffering from ocular photosensitive pathology or using photosensitive drugs, etc.);
- some directional LED lamps sold for home applications, if viewed at distances equal or shorter than 200 mm;
- the prolonged and repeated use of cold white LED-based devices by children and teenagers, especially in the evening, etc.
Individual Characteristics
Each person is unique. We do not respond to equal light exposure the same way. Genetics, morphology, ethnics, gender, age, behaviors (smoking, diet, etc.), squinting effects, eye protection (eyewear, shadow cap, nutraceuticals, etc.) are all contributors to a distinct personal risk profile.
Age, for instance, is largely involved in the progressive deterioration of visual functions such as dark adaptation. These findings are supported by the histological observations that rods degenerate early in both ageing and AMD. With age, the number of photosensitizers is rapidly increasing in the retina, particularly in the RPE where the lipofuscin age pigment builds up. This increase is partly due to blue photo-ageing processes. Age plus cumulative blue light exposure may irreversibly alter the classical visual cycle in the outer retina, progressively leading to AMD.
New tools for personalized early diagnosis
In the case of AMD, Erica Fletcher et al. have interestingly reviewed the new means of detecting the signs of early-stage disease. AMD has genetic and environmental risk factors. Genetic testing is now readily available using a combination of 16 genes to help predict the risk profile of an individual. Among the genes associated with an increased risk of developing AMD is complement factor H (CFH), the mutations of which contribute to explain immune dysregulation in AMD. Considering retinal imaging, Hogg et al. have recently highlighted that a particular form of drusens called reticular pseudo-drusen, at a subretinal level, is a risk-factor for progression to late-stage disease. A research team in the Netherlands has developed a software to analyze color fundus photographs so as to quantify and characterize drusens and determine a risk assessment. Using fundus autofluorescence (FAF), the RPE cell dysfunction can be detected through the accumulation of lipofuscin. Some scientists report that certain patterns in FAF imaging can help predict the evolution of AMD towards the choroidal neovascularization (CNV) late-stage form. Psychophysical methods can also help define if a subject is at greater risk of AMD or, later, of progressing to late-stage disease. Among those methods, dark adaptation disruption is an early marker of the disease. Next, it would be helpful to monitor the light exposure in cohorts of patients and try to associate disease progression. Therefore, a comprehensive clinical assessment and biomarker testing, including questionnaires (family history, lifestyle, etc.), visual examination and specific psychophysical methods, imaging, genetic testing and light exposure profiling can define an individual profile risk and help detect and characterize AMD at an early stage as well as monitor disease progression.
3. PERSONALIZED PREVENTION
For most eye diseases, an individual can undergo a complete assessment of his or her risk profile specifically related to a disease and then if needed, once informed and educated, decide to adopt a more thorough and frequent medical and self-surveillance so as to detect early enough the onset and progression of a disease and to take personalized prophylactic actions.
Light protection should be part of a personalized prophylactic program instructed by eye care practitioners. If a patient is profiled at risk of one of the vision-threatening diseases, and if the precise light spectrum incriminated as a risk factor is known (such as blue-violet light for AMD), then it is in the patient’s interest to protect himself selectively, especially when a portion of visible light needs to be filtered out as part of the preventative measure. Contrary to UV radiations, which can be fully blocked with no compromise on vision, filtering out visible light is always a trade-off with color vision and other physiological functions such as chronobiology or scotopic vision. Fortunately, the ophthalmic optics industry, in recent years, has fostered or benefited from the emergence of innovative narrow-band filtering technologies and has now started to develop photoselective and photoprotective ophthalmic filters. These new lenses offer practitioners an effective complementary tool to the armamentarium of prophylactic solutions available to their patients.
4. CONCLUSION
Major eye diseases, such as cataracts, AMD, glaucoma or diabetic retinopathy, have a tremendous impact on patients’ quality of life but also significant implications on the cost of healthcare. The threat is enhanced by the accelerated ageing of the world population and by evolving behavioral and environmental factors. Photobiology research has progressively identified light as a risk factor for these multifactorial conditions. Further photobiology studies, in vitro and in vivo, with well-calibrated light conditions, along with epidemiology studies with proper spectral light exposure quantification, are now necessary to identify significant correlations between light action spectra and pathogenic mechanisms. As initiated in RPE and RGC models, Essilor and the Paris Vision Institute are pursuing their efforts in developing accurate photometric tools (devices and protocols) for controlled cell illumination. A better understanding of the pathogenesis of eye conditions would interestingly be complemented by a better disease management, including real-life phototoxicity studies to find light-specific early markers of a disease and individual risk profiling. In our digital world with huge technical tools in image analysis and miniaturized sensors, the personalized and early follow-up of an eye disease is now made possible and paves the way for relevant personalized prevention. In the meantime, technology breakthroughs in the ophthalmic optics industry are meant to provide new effective solutions to design efficacious and personalized photo-protective and photo-selective eyewear.
Article from the magazine "Point de vue"


